I wanted to provide a recent research round-up on the connection of MS to vascular health, specifically how endothelial dysfunction contributes to MS progression.
As I've explained in another blog post, I do not feel it is appropriate for me to be dispensing medical advice as a layperson. But I am still interested in seeing the exploration of the vascular connection to MS, and happy to share that the research continues.
Jeff is now 15 years out from his MS diagnosis, and has shown no further MS disease progression. His most recent MRI from last June shows continued healing of old lesions and completely healthy, normal gray matter. And he's still composing, conducting, traveling, teaching, biking, jogging, living. As his new GP recently commented, he's an incredibly healthy 59 year old man. The fact that he has had MS for 15 years astonishes her. Me too.
Here's a short research wrap up:
1. A group of Japanese neurologists examined the level of endothelial dysfunction exhibited by people with MS and found a correlation between lower flow mediated dilation (FMD), endothelial dysfunction, and MS progression. FMD is lowered when we do not have enough endogenous nitric oxide.
Twenty-seven patients with MS and 24 healthy controls were enrolled. FMD was significantly lower in MS subjects than in control subjects (6.0 ± 0.6 vs. 8.6 ± 0.7, p = 0.006); furthermore, BHI was similarly lower in MS than in controls, but insignificant. Remarkably, FMD was significantly lower in secondary progressive MS subjects than in relapse-remitting MS subjects (3.7 ± 1.3 vs. 6.7 ± 0.7, p = 0.045). In addition, FMD was inversely correlated with the disability score as per the expanded disability status scale (R2 = 0.170, p = 0.033) and modified Rankin scale (R2 = 0.187, p = 0.027).
https://www.msard-journal.com/article/S2211-0348(21)00402-8/fulltext#%20
2. Russian neurologists are looking at how homocysteine decreases endothelial health, and contributes to MS progression.
The effect of homocysteine on endothelial dysfunction was shown in vitro. It was reported that homocysteine (500 µM) decreases the viability and induces the apoptosis of human vascular endothelial cells. Increases in reactive oxygen species in endothelial cells treated with homocysteine were also found [70]. Increasing concentrations of reactive oxygen species in endothelial cells homocysteine may induce vascular inflammation [19,28].
https://www.mdpi.com/2076-3425/10/9/637/htm
I first wrote about the danger of elevated homocysteine levels as part of the Endothelial Health Program
Anemia/low vit. B12 creates high levels of homocysteine in the blood (a sulfur containing amino acid) which damages the endothelium A strict vegetarian diet that excludes all meat, fish, dairy and eggs, or an unbalanced diet of processed foods could create low vit. B12 levels and damage the endothelium (10)
https://ccsviinms.blogspot.com/2016/04/the-endothelial-health-program-8-years.html
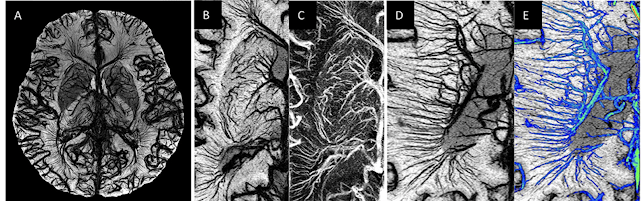
3. Our friends from the ISNVD, Dr. Mark Haacke and Dr. Yulin Ge, continue to look at the MS brain, to study the vasculature. This group is noting a new venous vascular sign in lesions, which they are calling multiple vessel sign (MVS). Using a new contrast agent called Ferumoxytol, the researchers were able to see enhanced images of MS lesions, and noticedmany more small vessel abnormalities. Rindfleisch's discovery of the dilated blood vessel, the central vein sign, continues to be explored.
The total number of lesions with vascularity on pre- and post-contrast data were 287 and 488, respectively. The lesions with abnormal vascular behavior were broken up into following categories: small lesions appearing only at the vessel boundary; dilated vessels within the lesions; and developmental venous angiomas. These vessel abnormalities observed within lesions increased from 55 on pre-contrast data to 153 on post-contrast data. Finally, across all the patients, the periventricular lesional vessel density was significantly higher (p < 0.05) than that of the periventricular NAWM.
https://pubmed.ncbi.nlm.nih.gov/33338965/
4. And finally, here is a fabulously thorough review from the ISNVD 2020 meeting, published in 2021. This paper can serve as a primer for any medical researcher interested in learning more about the vascular connection to MS. It's all here.
https://www.frontiersin.org/articles/10.3389/fneur.2021.561458/full
stay well,
Joan